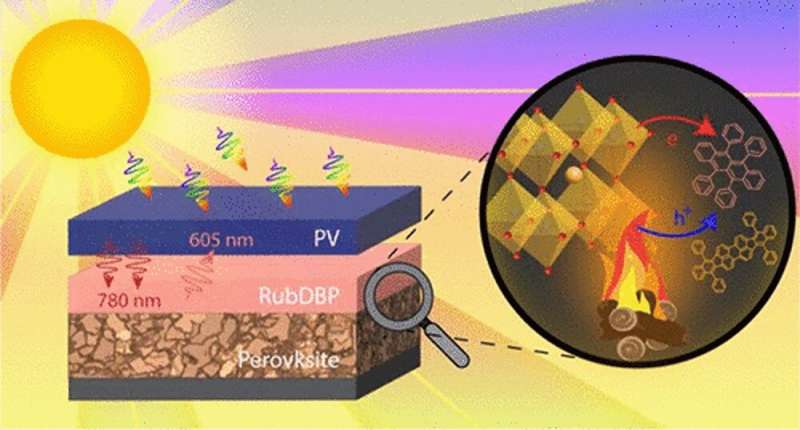
Graphical Abstract. Credit: The Journal of Physical Chemistry C (2023). DOI: 10.1021/acs.jpcc.2c08850
Researchers at Florida State University and the FAMU-FSU College of Engineering are helping build the solar cells of tomorrow by examining how a next-generation material can operate efficiently under real-world conditions that include baking temperatures and hours of sunlight.
Their work was published in The Journal of Physical Chemistry C.
Most solar cells are made of silicon, but ongoing research is looking at other options, including a material known as perovskite. In addition to acting as a solar cell, perovskites can also enable a phenomenon called upconversion in organic molecules, a process in which the perovskite absorbs low-energy photons and the organic molecules then convert these particles into high-energy photons.
"We wondered if there was another way of using the photons—the particles of energy in light—that otherwise would not be converted into electricity?" said Theo Siegrist, a professor in the Department of Chemical and Biomedical Engineering. "We want to store the energy from one photon until a second comes around and combine the two photons into one that can overcome the barrier."
"The idea is that one usable high energy light particle is emitted again after the upconversion process," said Lea Nienhaus, assistant professor in the Department of Chemistry and Biochemistry.
Previous research into perovskites examined how high temperatures and light degrade them but hadn't considered the upconversion process in perovskite/organic bilayers under real-world conditions. Understanding how these devices work in typical heat and light conditions shows researchers where to direct their efforts for implementation into commercial solar cells.
The researchers placed their upconversion devices on a heating element, raising their temperature to about 60 degrees Celsius. Then they used optical spectroscopy and X-ray crystallography to examine their properties.
They found that the performance of the upconversion device was significantly diminished after exposure to high temperatures, but not because of perovskite degradation. Instead, the organic molecules required for the upconversion process crystallized under the heat, rendering the device ineffective.
"The perovskite on its own, if you heat it and shine light on it, it degrades," Niehaus said. "As soon as you put the organic molecules on top, it no longer degrades. The organic molecules are contributing to a more long-lived perovskite, which I think is a very useful result. There are still engineering issues to be solved to make perovskite-based upconversion devices viable, but our hope is that this work is part of addressing those issues."
More information: Alexander S. Bieber et al, Perovskite-Sensitized Upconversion under Operando Conditions, The Journal of Physical Chemistry C (2023). DOI: 10.1021/acs.jpcc.2c08850
Journal information: Journal of Physical Chemistry C
Provided by Florida State University