Scientists from Nanjing University of Posts and Telecommunications developed SSSMBs using a high energy Na4MnCr(PO4)3 cathode. Credit: Zhongyue Wang, Nanjing University of Posts and Telecommunications
A team from China has broken new ground in the development of sodium ion batteries (SIBs.)
"The development of high safety and high energy density SIBs is imperative," said paper author Zhongyue Wang, lecture with the College of electronic and optical engineering & college of flexible electronics (future technology), Nanjing University of Posts and Telecommunications. "Currently, great progress has been made in sodium-ion batteries, but their energy density is still much lower than LIBs limited by the cathode."
Wang explained that NASICON-type phosphate (NaxMM'(PO4)3, M, M' =transition metal Ti, V, Cr, Mn, Fe, Co and Ni) is regarded as the most competitive high energy cathode benefiting from its stable structure, 3-D open frameworks and structural diversity.
"NaxMM'(PO4)3 contains an enormous Na reservoir with potential for up to four extractable Na; however, it is uncommon to perform reversible extraction/insertion of more than two Na ions," Wang said. "One/two-electrons electrochemical reaction per formula unit makes most phosphate cathode provide low energy density (<500 Wh kg-1). Hence, exploring a cathode enable three-electron reaction and high operating voltage is extremely desirable to achieve a higher energy density for SIBs."
But not all NaxMM'(PO4)3 cathodes are capable of three-electron reactions. According to Wang, you can't have your cake and eat it. Cheap Ti and Fe -based phosphates cathodes can achieve a specific capacity of over 170 mA h g-1, but the lower operating voltage results in a low energy density.
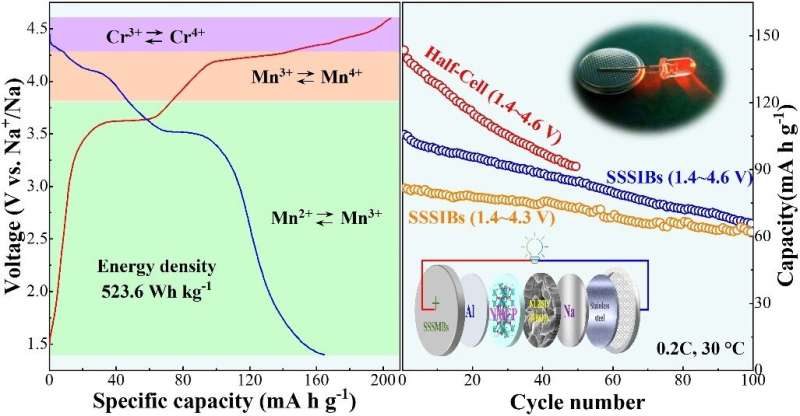
On the other hand, extra-high voltage plateau of Co or Ni-based phosphates cathodes (over 4.5 V vs Na+/Na) poses a great challenge to the electrochemical stability of electrolyte and cause its decomposition inevitably. Na4MnCr(PO4)3, due to its higher voltage plateau (4.34 V for Cr4+/3+, 4.11 V for Mn4+/3+, 3.43 V for Mn3+/2+), high theoretical energy density (566 Wh kg-1), low cost and low/non-toxic, will become an ideal cathode for SIBs.
However, there are a few reports on Na4MnCr(PO4)3, the results are very inconsistent and the cycle performance over 4.5 V cutoff voltage windows is very poor. Wang said that the main reason is the decomposition of organic electrolyte under high voltage. Wang and his team synthesized carbon coated high energy Na4MnCr(PO4)3, and more importantly, realized better cycle stability in solid state sodium metal batteries.
"In this paper, the surface carbon content of Na4MnCr(PO4)3 was controlled by adjusting the molar ratio of citric acid and metal ions, and the electrochemical performance of Na4MnCr(PO4)3 was optimized, an ultra-high energy density of 523.6 Wh kg-1 was obtained," Wang said. "The as-prepared Na4MnCr(PO4)3@C were assembled into solid state sodium metal batteries by matching Na3.3La0.3Zr1.7Si2PO12(3 wt% Na2B4O7) ceramic electrolyte, and achieved a more stable cycle than that of half-cell."
"Better cyclic stability is attributed to the super high ionic conductivity (1.2 mS cm-1) of Na3.3La0.3Zr1.7Si2PO12(3 wt% Na2B4O7) and its wide electrochemical stability window (>5 V)," Wang said.
"There is still a lot of room for improvement in Na4MnCr(PO4)3-based solid-state batteries," Wang said. "For example, ultra-thin composite solid electrolyte is expected to improve the interface issues and increase the energy density of SSSIBs, and finally to realize the practical applications."
The findings are published in the journal Energy Material Advances.
More information: Zhongyue Wang et al, High-Energy Na4MnCr(PO4)3@C Cathode for Solid-State Sodium Metal Batteries, Energy Material Advances (2023). DOI: 10.34133/energymatadv.0036
Provided by Beijing Institute of Technology Press Co., Ltd