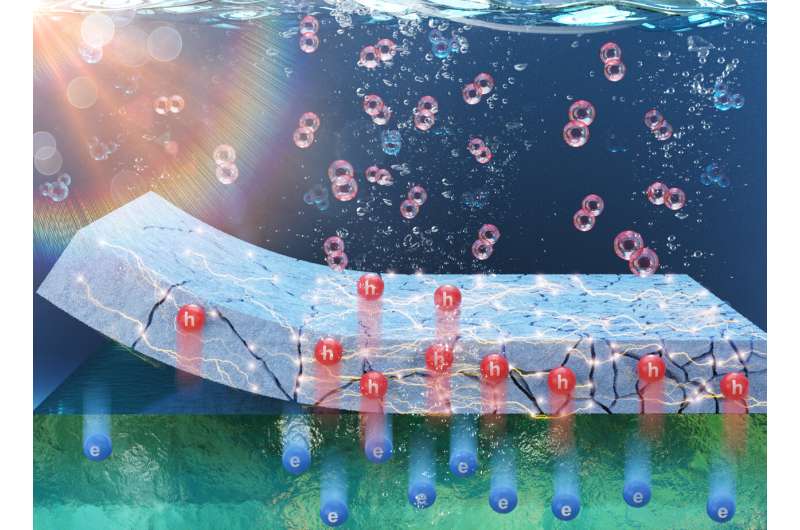
Highlighting a study on a mechanism of photoelectrochemical water splitting on Si photoanode passivated with TiOx layer with various defect density from the laboratories of Dr. Ansoon Kim at Korea Research Institute of Standards & Science (KRISS). Credit: Korea Research Institute of Standards and Science (KRISS)
Hydrogen has been gaining attention as a clean and efficient energy source. However, is hydrogen really environmentally friendly? Most hydrogen commonly used now is gray hydrogen derived from fossil fuels. Since its production process accompanies generation of green house gas, it can be said that gray hydrogen is not environmentally friendly in the strict sense. The era of green hydrogen without carbon emissions has not yet begun.
The Korea Research Institute of Standards and Science (KRISS) has demonstrated the key to the longevity and efficiency of a photoanode with protective film, which is used to produce hydrogen via water splitting using solar energy. This is expected to bring forward the era of environment-friendly green hydrogen.
Green hydrogen is produced without carbon emissions by using renewable energy sources. A representative method to produce green hydrogen is photoelectrochemical water splitting using photoanode which is directly immersed in electrolyte and can absorb sunlight. As a result, the photoanode directly splits contacting water into hydrogen and oxygen using absorbed solar energy. However, as the photoanode is in direct contact with electrolyte, it is prone to surface corrosion. Surface protective coatings were deposited on the surface to prevent surface corrosion.
Typically, oxide materials such as titanium dioxide (TiO2) are used as protective films for photoanodes. Although oxide materials are poor conductors of electricity, their conductivity can be modulated when oxygen defects, serving as a channel for charge transport, are formed. The key to extending the lifespan of photoanodes is to develop a protective film durable enough to prevent electrode corrosion and capable of maintaining optimal electrical conductivity.
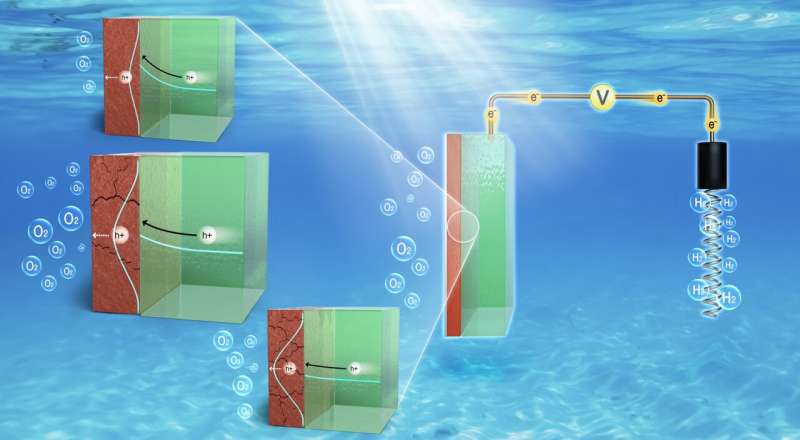
For the efficient PEC water splitting, it is crucial to balance two factors by systematically controlling the defect density in TiOx passivation layer of n-Si photoanode, which are (1) accessible density of state for carrier transportation in forbidden gap and (2) favorable interface energetics. Credit: Korea Research Institute of Standards and Science (KRISS)
KRISS has developed the world's first technology for systematically modulating the levels of oxygen defects in a titanium dioxide (TiO2) protective film of photoanode to maximize hydrogen production efficiency. In order to explore the role of oxygen defects in the charge transfer mechanism, the research team determined the optimal levels of defects that maximize photoanode lifespan and hydrogen production by using X-ray photoelectron spectroscopy and electrochemical analysis
Unlike past studies that relied on spontaneously formed oxygen defects in the protective film during manufacturing process, this research proposes a direct method of production that controls the levels of oxygen defects, enabling mass production.
According to the experimental results, the photoanode without a protective film showed a rapid degradation in lifespan within an hour, causing the hydrogen production efficiency to fall below 20% compared to the initial state. On the other hand, the photoanode with optimized protective film maintained a hydrogen production efficiency of over 85% even after 100 hours.
This achievement has the potential to enhance the efficiency and lifespan of photoanodes and can be applied to other clean technologies that rely on photoanodes. The artificial photosynthesis technology that captures carbon dioxide and converts it to a chemical energy source using solar energy is one of the examples.
Dr. Ansoon Kim, a principal researcher at KRISS Interdisciplinary Materials Measurement Institute, said, "This approach can improve photoanode lifespan by approximately 10 times and significantly contribute to the commercialization of green hydrogen."
KRISS plans to conduct further research to unveil the optimal levels of oxygen defects and underlying principles that maximize the lifespan of photoanodes.
The research is published in the Journal of Materials Chemistry A.
More information: Songwoung Hong et al, Role of defect density in the TiOx protective layer of the n-Si photoanode for efficient photoelectrochemical water splitting, Journal of Materials Chemistry A (2023). DOI: 10.1039/D2TA07082K
Journal information: Journal of Materials Chemistry A
Provided by National Research Council of Science & Technology