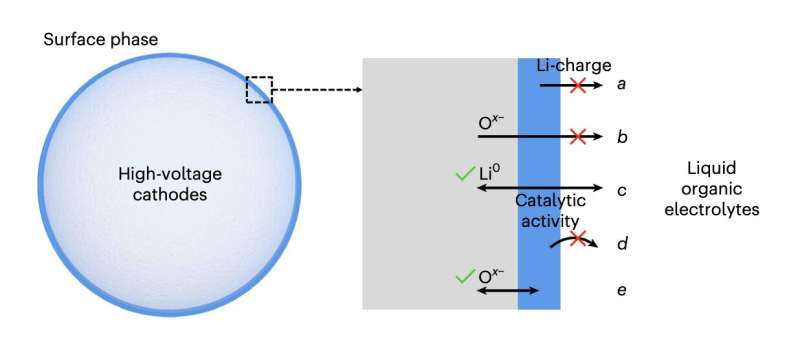
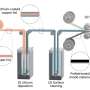
The ideal surface phase should satisfy the following design criteria: a, Li-charging inactive; b, kinetically shut-off oxygen (Oα− , 0≤α ≤ 2) outgassing from cathode to organic electrolytes; c, a good mixed ionic and electronic conductor to facilitate ambipolar Li diffusion; d, catalytically inactive and with strongly bonded surface oxygen; e, be able to encapsulate labile oxygen given out by highly delithiated cathode. Credit: Nature Energy (2023). DOI: 10.1038/s41560-022-01179-3
To support the operation of the countless electronic devices that are being developed every day, researchers will need to develop increasingly advanced battery technologies. Lithium-ion batteries (LiBs), some of the most employed rechargeable batteries worldwide, still have significant room for improvement.
LiBs are batteries that contain one or more lithium-ion cells, along with a protective circuit board. In these batteries, lithium ions move between a cathode (i.e., positive electrode) and an anode (i.e., negative electrode), while electrons move in the opposite direction within the batteries' external circuit.
While LiBs are now widely used worldwide, they still have some notable limitations. For instance, high-energy-density cathodes in LiBs are known to be vulnerable to labile oxygen losses and fast degradation. This can significantly limit the stability and safety of some lithium-based batteries, increasing the risk of interactions between oxides or allied oxygen radicals with organic electrolytes in the batteries.
As a result, some battery developers have suggested that the use of high-energy cathodes in LiBs is generally unsafe and undesirable. A paper by researchers at Peking University, Tsinghua University, the Chinese Academy of Sciences, and Massachusetts Institute of Technology (MIT) introduces a strategy that could help to overcome the challenges associated with the use of high-energy cathodes in LiBs. The research is published in the journal Nature Energy.
"We develop the theory underlying the high-voltage-induced oxygen evolution crisis and report a lanthurizing process to regulate the near-surface structure of energy materials beyond conventional surface doping," Mingzhi Cai, Yanghao Dong and their colleagues wrote in their paper. "Using LiCoO2 as an example and generalizing to Co-lean/free high-energy-density layered cathodes, we demonstrate effective surface passivation, suppressed surface degradation and improved electrochemical performance."
Essentially, Cai, Dong and their colleagues created a perovskite surface layer with a high electronic conductivity but a low oxygen ion conductivity. They then showed that this layer could act as an oxygen "buffer," kinetically inhibiting the oxygen evolution reaction (OER) while preserving the correct functioning of high-energy cathodes in LiBs.
The approach used by the researchers is based on a so-called lanthurization process. This process was found to successfully stabilize high-voltage cycling in high-energy-density LiBs, improving their safety and stability.
"High-voltage cycling stability has been greatly enhanced, up to 4.8 V versus Li+ /Li, including in practical pouch-type full cells," Cai, Dong and their colleagues wrote in their paper. "The superior performance is rooted in the engineered surface architecture and the reliability of the synthesis method. The designed surface phase stalls oxygen evolution reaction at high voltages."
The recent study by this team of researchers unveils new processing opportunities for the engineering and coating of surfaces via high-oxygen-activity passivation, selective chemical alloying and strain engineering. The strategy they developed could ultimately inform the development of both LiBs and other battery solutions that are more stable and reliable, particularly during high-voltage cycling.
More information: Mingzhi Cai et al, Stalling oxygen evolution in high-voltage cathodes by lanthurization, Nature Energy (2023). DOI: 10.1038/s41560-022-01179-3
Journal information: Nature Energy
© 2023 Science X Network