This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Scientists developing way to make cheaper lithium batteries with sodium

Lithium is becoming the new gold, with rocketing use in lithium-ion batteries in electric cars, computers, and portable devices driving up the price and affecting the supply of the relatively rare metal. Scientists are on the verge of developing a way to use sodium to replace some of the lithium, so driving down costs and guaranteeing the supply.
Recently scientists have looked at dispensing with lithium altogether and instead using sodium or other elements in high quality batteries. Sodium is cheaper and more available (it's found in seawater, as sodium chloride), but it has disadvantages, and lithium batteries remain the best in terms of delivering the concentrated charge needed to power cars and portable devices.
Ph. D student Tullio Geraci and Professor Alexandra Navrotsky from Arizona State University have adopted a different approach; mixing lithium and sodium in the same battery promises to ease supply problems and pave the way to cheaper batteries and a more secure supply chain.
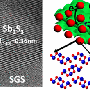
The group is making lithium-sodium materials and characterizing their structures, homogeneity, and thermodynamic properties. The researchers use a specialized technique developed and optimized in the Navrotsky laboratory (high-temperature oxide melt solution calorimetry) to measure the energetic stability of the materials, while heating experiments determine their possible decomposition in use.
Presenting their findings at Goldschmidt 2023 in Lyon, an international geochemistry conference, Geraci said "We have been mixing small amounts of sodium with lithium, and testing it for stability, and then seeing how it performs. It's a step-by-step process, and when we first started the stability was not promising—the first thing we need is to see if the mixture stays in a usable form. But as we increased the sodium content stability improved. So far, we have achieved a 10% mixture, and it seems fine, it's still thermodynamically stable. We believe can push this up to around 20% before we see any significant difference in performance."
Geraci continued, "At first we were unsure whether these Li/Na dilutions could even be made. Surprisingly, we found that weak dilutions tend to break down, the solutions lose their homogeneity and the crystal structure which is important to produce a battery. But as we increase the amount of sodium the material becomes more stable. After we have arrived at the optimum mix, we need to turn our findings over to battery technologists to produce the first sodium-lithium batteries. We believe these are the first steps in developing a new battery technology."
Professor Nancy Ross (Department of Geosciences, Virginia Tech, Blacksburg VA) said, "The research by Geraci and Navrotsky highlights how geochemistry can be applied to developing new materials of technological importance. Their research opens a promising avenue to explore alternative, more affordable and sustainable sources for Lithium batteries that we depend upon in our daily lives."
More information: conf.goldschmidt.info/goldschm … /2023/meetingapp.cgi